Pharmacogenomics: towards the personalised medicine of the future

Let’s think about the future. Imagine you are visiting your doctor, who analyses your genetic biomarkers to prescribe you the most suitable medicine, at the right dose, at the right time.
This could happen before you expect it, and it would solve some of the main issues the healthcare industry is currently facing.
For instance, adverse drug reactions cause a considerable increase in morbidity and mortality rates. It has been estimated that it produces approximately 5% of all hospital admissions and 197,000 deaths annually throughout Europe, and implies a total cost of around 79 billion euros for the healthcare systems.
But what is the reason for the ineffectiveness of certain medicines? Drugs are generally tested on many people, and the average response is analysed to decide the adequate dose for most cases. This sort of evidence-based medicine relies on averages’ law, so the prescription doesn’t consider the specific genetic variation between humans.
In that way, this situation becomes an absolute priority not just for the healthcare industry or entrepreneurial R&D start-ups but also for official institutions like the European Commision to study personalised treatment prescriptions.
In this context, Pharmacogenomics arises: the method that recognises that two patients are not alike. What are the main characteristics of this field, and why is it crucial in terms of innovation?
Pharmacogenenomics as an essential tool
According to the European Medicines Agency, Pharmacogenomics studies “variations in DNA sequence as related to drug response”. In other words, this science analyses the relation between the patient’s genetic polymorphisms and the drugs prescribed and the interactions between the whole medication. In this manner, it aims to improve the medical decision-making process and rational drug prescription to benefit both the patient and the national healthcare system.
Based on an individual’s genetic constitution, pharmacogenomics allows differentiating responders from non-responders to medications. Responders metabolise the drug too slowly, so they can suffer from drug toxicity and need a lower dose of the medicine. In comparison, non-responders can metabolise the drug very quickly, so it doesn’t cause any therapeutic effect and a higher dose is required.
Pharmacogenomics can also consider drug-drug interactions, thus would have a tremendously beneficial impact on elderly patients who are usually polymedicated. Therefore, thanks to the clinicians’ ability to make more informed decisions, pharmacogenomics enables patients to get better-targeted therapies causing a higher probability of desired outcomes and a reduced likelihood of adverse reactions to medicines.
When increasing healthcare costs are becoming a significant problem, pharmacogenomics can personalise treatments, resulting in improved quality of life and lowered healthcare expenditure. In this context, this science reduces hospital admissions by adverse drug effects and implements prevention treatments according to the patient’s genetic risk to a specific disease.
The European role in the progress of personalised medicine
Historically, the limiting factor for implementing targeted medicine came from the sequencing cost and the volume of data generated. But the technological breakthroughs are making price no longer a problem, which is why this discipline has already emerged.
Over the last years, several countries have implemented strategies and plans for Personalised or Genomic Medicine at a national level. The Personalised Medicine Action Plan (Germany), Genomic Medicine 2025 (France), Spanish Strategy for Personalized Medicine (Spain) and Finland’s Genome Strategy (Finland) are just some examples.
From a European level, we can also find some actions to contribute to personalised medicine implementation. Some of the most relevant ones are included below.
Horizon 2020
Even though it has already ended, the last EU Research and Innovation programme Horizon 2020 (2014-2020) has played its part in supporting personalised medicine by funding the most innovative projects in that field. Indeed, the advisory group to the health, demographic change, and well-being societal challenge of Horizon 2020 has highlighted this science as a key aspect to be addressed during the Work Programme.
One of the most ambitious initiatives received a €15M grant through the RIA (Research and Innovation Action) H2020 Programme. The Ubiquitous Pharmacogenomics Consortium (U-PGx) is the first large-scale, international pharmacogenomics project created to implement pharmacogenomics tests for a broad range of drugs and assess this new science’s impact on health. The assessment outcomes will provide regulatory authorities with evidence on both the patient and the economic impact of pharmacogenomics to enable informed-based decision-making to shape its implementation policy.
Another relevant one is ICPedMed, a platform supported by over thirty European and international members to exchange information on personalised medicine funding, implementation, and research.
This initiative, in which the European Commission acts as an observer, supports this science activity to agree on future research actions at an international level. In this way, the ICPedMed works to transform Europe into a global leader in this area and, ultimately, apply this field to citizens’ lives and treatments.
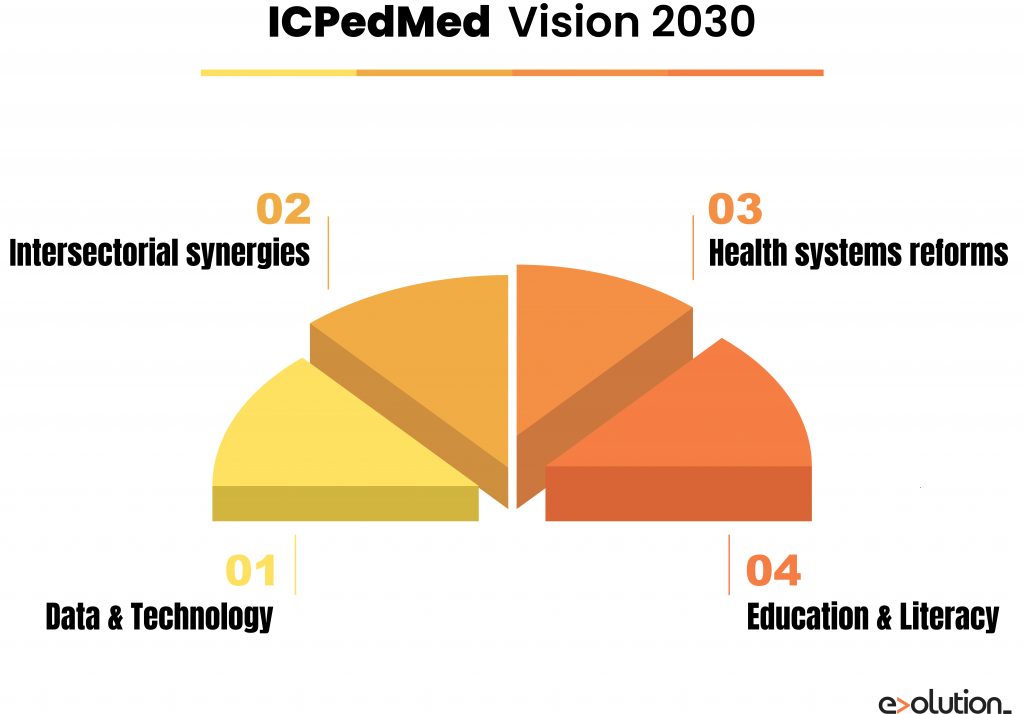
The ICPerMed has developed a Vision Paper of how personalised medicine methods will promote “next-generation” medicine in 2030. Four pillars representing transversal issues are crucial for the successful implementation of personalised medicine by 2030:
- Data and technology: It’s needed to deploy innovative and flexible ICT solutions and make significant investments in artificial intelligence methods to integrate and interpret multilevel data from multiple sources.
- Inter-sectoral synergies: A close alignment between healthcare providers and research will facilitate a fast turnaround of research results for clinical implementation.
- Health systems reforms: These systems need to shift the focus from treatment to prevention, to make significant investments in centralised data infrastructure and digital platforms, to control GDPR concerning the private genetic information cautiously, and to create new jobs for data-related professions like “Information/Data Technicians”.
- Education and literacy: Clinicians, nurses and pharmacists will have to acquire the required skills to understand biomarker information and improve their digital knowledge. Healthcare managers and policymakers will have ample evidence of personalised medicine’s benefits to establishing political frameworks regulating its implementation, for example, by forcing drug labelling with pharmacogenetics information.
Horizon Europe
This concern about the continuous improvement of the healthcare system reaches Horizon Europe (2021-2027). Even the European Commission is still working on this new programme, some of its characteristics have been released. It is the case of its missions and pillars.
As we have mentioned in previous articles, the first goal is to achieve science excellence. Under this objective, Horizon Europe will enhance the Union’s science and consolidate the European Research Area to make the Union’s R&D system more competitive globally. This pillar is also related to one of the main Horizon Europe missions: mitigating cancer through better diagnosis and treatments.
According to Europe’s Beating Cancer Plan, personalised medicine is one of the latest health advances that have changed patients’ condition. Thus, medical projects and initiatives submitted to Horizon Europe may get more possibilities to successful funding options if they pursue this kind of research, which is crucial for the Commission.
On the other hand, because of the current pandemic, Horizon Europe would pay special attention to developing personalised medicine to understand better the individual host response to viruses, like COVID-19.
Furthermore, this programme would help patients who suffer from protective or harmful immune responses by studying biomarkers, developing personalised therapeutic interventions and vaccines, and improving disease prevention and risks.
What are the next steps for Pharmacogenomics?
Pharmacogenomics is one of the essential tools future medicine will count on to improve predictive and preventive medicine. Personalised medicine will be based on using an individual’s genetic profile to make the best therapeutic choice by facilitating predictions about whether that person will benefit from a particular treatment or suffer serious side effects.
Although some organisations and institutions like the European Commission are trying to implement this kind of healthcare research, we are still at an early stage. The translation of results into a benefit for patients has been limited yet.
We should not forget the last objective of Pharmacogenomics: saving and improving patients’ lives. Advantages are numerous but, still, R&D is necessary to solve all the questions and doubts to enhance personalised medicine and healthcare. In this way, initiatives that contribute to improving this situation could access better opportunities and funding benefits.
And now, can you imagine that visit to the doctor again? Let’s work together to make that happen.
Do you have an R&D project that will revolutionise the healthcare industry? We want to meet you!
